Abstract
Background: Bruton's tyrosine kinase inhibitors (BTKi) are highly effective in relapsed/refractory (r/r) mantle cell lymphoma (MCL) but have a progression-free survival (PFS) of roughly 2 years. Proteasome inhibitors are active in r/r MCL, although the single-agent activity of bortezomib is modest (ORR 33%), and administration is limited by peripheral neuropathy. Ixazomib (Ixa) is an oral proteasome inhibitor with a lower incidence of peripheral neuropathy. We evaluated the safety and efficacy of ixa and ibrutinib (ibr) in r/r MCL.
Methods: Patients (pts) with r/r MCL ≥18 years old were eligible. Both ibr-naïve and ibr-pretreated pts were eligible for phase 1. Pts were divided into two cohorts (ibr-naïve and ibr-pretreated) for the phase 2 portion. The ibr-pretreated cohort was closed early due to slow enrollment. We evaluated 2 dose levels (DLs) of ixa (3mg and 4mg, days 1, 8, and 15 of a 28 day cycle) in the phase 1 study, and all pts received ibr 560mg daily. Treatment continued until disease progression or unacceptable toxicity. Pts were enrolled in a 3+3 manner, and the 4mg dose level was expanded to 6 pts evaluable for dose limiting toxicity (DLT). Pts were monitored for DLT during cycle 1, defined as grade 3 thrombocytopenia with significant bleeding, select grade 3 non-hematologic toxicities, grade 4 thrombocytopenia, grade 4 febrile neutropenia, grade 4 non-hematologic toxicity, or any grade 5 toxicity. In addition, any toxicity-related dose delay > 7 days of ibr or ixa or an inability to receive all 3 doses of ixa during cycle 1 were considered DLT's.
The primary endpoint for the phase 2 study was complete response (CR) within the first 12 months of study treatment. We targeted a CR rate of 40% (based on a historical CR rate of 21% with ibr monotherapy) for the ibr-naïve cohort. We planned for a sample size of 31 pts per cohort, and ibr-naïve pts treated at the RP2D in the phase 1 study were counted for the phase 2 primary endpoint.
Results: 43 pts were enrolled to the study overall, with a median age of 70 (range 52-84) and 74% were male. Twelve pts were treated in the phase 1 study (including 3 pts treated at DL1 and 9 at DL2). Eleven pts enrolled to the phase 1 study were ibr-naïve and 1 was ibr-pretreated. Three pts enrolled at DL2 were replaced during the phase 1 study due to growth factor administration (n=1) and difficulty adhering to the therapy administration schedule (n=2) and were not evaluable for DLT. One pt treated at DL2 experienced a grade 3 lung infection that was classified as a DLT, while the other 5 evaluable pts did not experience a DLT. As a result, DL2 was the RP2D.
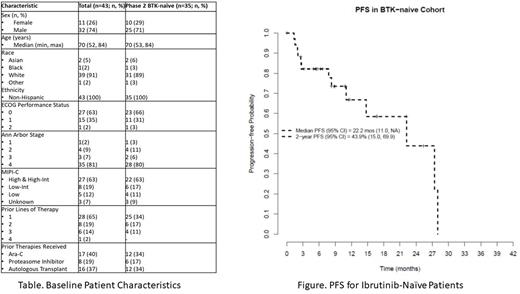
Thirty-five ibr-naïve pts were treated at the RP2D. Prior lines of therapy were 1 (n=25), 2 (n=6), and 3 (n=4). Remaining baseline characteristics are summarized in the Table. The combination was active with a CR rate (95% CI) of 42.9% (26.3, 60.6), and ORR of 77.1% (59.9, 89.6). The median duration of treatment is 8 cycles (range: 1-23), with 8 of 35 (23%) pts remaining on treatment after a median of 12 cycles (range: 6-19). Reasons for treatment discontinuation for the remaining 27 pts were AE (n=13), progression (n=9), death due to AE, MD discretion, and treatment delay > 21 days (n=1 each). Pts who discontinued therapy due to AE's received 1-2 cycles (n=4), 3-6 cycles (n=4), or 7+ cycles (n=5). With a median follow-up of 16.8 months; the 2-year PFS was 44%, and the 2-year OS was 91% (See Figure). Median duration of response was 16.7 months.
Grade3+ AE's occurring in > 1 patient included: hypertension, lymphopenia, neutropenia, thrombocytopenia, rash, syncope, and atrial fibrillation. One pt with known cardiovascular comorbidities experienced a grade 5 aortic injury that was deemed possibly related to ibrutinib. AE's leading to treatment discontinuation included: rash (n=2), atrial fibrillation (n=2), hepatic failure, sepsis, fatigue/anorexia, peripheral neuropathy, muscle spasms, arthralgia, thrombocytopenia, diarrhea, heart failure (n=1 each).
Conclusion: The combination of ibr and ixa resulted in a substantial improvement of CR rate beyond ibr monotherapy for ibr-naïve pts. However, with sustained treatment > 1/3 of pts required treatment discontinuation due to AE, some of which were likely related to ibr. PFS approximates that seen with ibr monotherapy. Further exploration of this combination may identify scenarios in which a fixed duration of treatment maximizes achievement of CR, which is important as a potential bridge to an alternative therapy.
Disclosures
Cohen:Janssen: Consultancy; HutchMed: Consultancy, Research Funding; Genentech: Research Funding; BMS/Celgene: Research Funding; Astrazeneca: Consultancy, Research Funding; Takeda: Research Funding; Kite Pharma/Gilead: Consultancy; BeiGene: Consultancy, Research Funding; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; Novartis: Research Funding; Aptitude Health: Consultancy. Hamadani:Kadmon: Consultancy; Kite: Consultancy; Gamida Cell: Consultancy; SeaGen: Consultancy; Omeros: Consultancy; Genmab: Consultancy; Takeda: Research Funding; Novartis: Consultancy; Legend Biotech: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy; MorphoSys: Consultancy; Incyte Corporation: Consultancy; Medical University of Wisconsin: Current Employment; Spectrum Pharmaceuticals: Research Funding; BioGene: Speakers Bureau; Astellas Pharma: Research Funding; Sanofi Genzyme: Speakers Bureau; AstraZeneca: Speakers Bureau. Diefenbach:BMS: Consultancy, Research Funding; Celgene: Consultancy; FATE Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Current equity holder in publicly-traded company; IMAB: Consultancy; Incyte: Research Funding; Kite: Consultancy; MEI Pharma: Research Funding; Merck: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Seattle Genetics: Research Funding. Landsburg:Morphosys: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Curis, Inc: Research Funding; Calithera: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Kahl:Incyte: Consultancy; Janssen: Consultancy; Kite: Consultancy; Beigene: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy; AcertaPharma: Consultancy; MEI: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Roche: Consultancy; ADT Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Hutchmed: Consultancy, Research Funding; TG Therapeutics: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy; Research To Practice: Speakers Bureau.
OffLabel Disclosure:
Ixazomib is not approved for mantle cell lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal